Introduction: The Chemistry That Shapes Our Lives
We live in a world built by organic chemistry—from the coffee that jumpstarts our mornings to the medicines that heal us, the clothes we wear, and even the screens we’re reading this on. But what exactly is this fascinating science, and why does it matter so much to our daily existence?
Organic chemistry is often perceived as a daunting subject, characterized by complex structures and reactions. But at its heart, it’s simply the study of carbon-based molecules—the very building blocks of life. More than just textbook knowledge, it’s a human endeavor shaped by curiosity, accidents, and the persistent drive to improve our world.
In this expanded exploration, we’ll uncover the human side of organic chemistry—the stories behind discoveries, the real-world applications that touch our lives, and why this science is far more accessible and engaging than its reputation suggests.
1. Carbon: The Element That Learned to Socialize
Why Carbon is Life’s Favorite Element
Carbon’s extraordinary ability to form diverse molecules comes down to its unique personality:
- Four bonding electrons allow it to form stable connections
- Flexible bonding styles (single, double, triple bonds)
- Talent for long chains and intricate rings
This versatility makes carbon the ultimate molecular architect. Just as a few musical notes can create infinite songs, carbon’s bonding patterns give us everything from simple methane to complex DNA.
The Vital Force Myth and Wöhler’s Accidental Breakthrough
The year was 1828 when Friedrich Wöhler, attempting to make ammonium cyanate, accidentally synthesized urea—a compound previously only known from living organisms. This serendipitous discovery:
- Destroyed the idea of “vital force” (the belief that organic compounds required life to form)
- Proved organic and inorganic chemistry follow the same rules
- Opened the door to synthetic organic chemistry
Today, we routinely create in labs what nature took millennia to evolve—from life-saving drugs to sustainable materials.
2. Functional Groups: Molecular Personalities
Understanding organic chemistry becomes easier when we recognize these molecular “character traits”:
The Molecular Cast of Characters
| Functional Group | Personality | Everyday Example |
| Hydroxyl (-OH) | The social drinker | Alcohol in cocktails |
| Carbonyl (C=O) | The sweet talker | Vanilla flavoring |
| Carboxyl (-COOH) | The sourpuss | Vinegar in salad dressing |
| Amino (-NH₂) | The bodybuilder | Proteins in your muscles |
| Aromatic rings | The perfumer | The smell of fresh coffee |
These groups explain why:
- Mint feels cool (menthol’s hydroxyl group)
- Chili peppers burn (capsaicin’s amide group)
- Your laundry smells fresh (esters in fabric softeners)
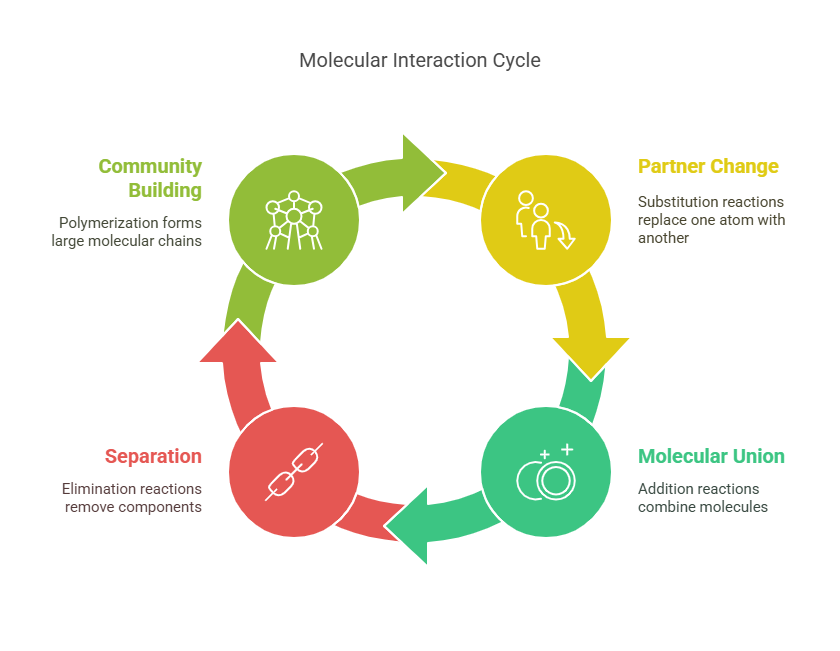
3. Chemical Reactions: Molecular Relationships
The Soap Opera of Molecular Interactions
Substitution Reactions: Like changing partners in a dance—chlorine replaces hydrogen in methane to form chloromethane (used in refrigerants).
Addition Reactions: The molecular equivalent of marriage—ethylene and water become ethanol (your favorite beer ingredient).
Elimination Reactions: The breakup—ethanol loses water to become ethylene (for making plastic bags).
Polymerization: Building communities—thousands of ethylene molecules join to make polyethylene (your milk jug).
Real-World Impact: From Lab to Life
- Haber Process: Fixes nitrogen from the air to make fertilizers, feeding billions
- Click Chemistry: 2022 Nobel Prize-winning method for building molecules like Lego
- Green Chemistry: Developing biodegradable plastics from plant sugars
4. Medicine: Where Molecules Meet Humanity
The Art of Drug Design
Creating medicines is like crafting molecular keys to fit biological locks:
- Aspirin: Modified from willow bark salicylic acid to be gentler on stomachs
- Penicillin: Discovered when mold contaminated a petri dish, now saves millions
- mRNA Vaccines: Organic chemistry made COVID-19 vaccines possible
Cancer Treatment Revolution
Modern drugs like Keytruda work like “molecular assassins”—targeting only cancer cells. Organic chemists design these precision weapons by:
- Understanding cancer’s molecular machinery
- Crafting compounds that disrupt only harmful processes
- Minimizing side effects through careful molecular tuning
5. Materials: Building the Modern World
From Silk to Synthetics
- Nylon: Born from a desire to replace Japanese silk during WWII, now in everything from stockings to parachutes
- Kevlar: Stephanie Kwolek’s accidental discovery while researching lightweight fibers
- Gore-Tex: Waterproof yet breathable, thanks to fluoropolymer chemistry
The Smartphone Revolution
Your phone contains dozens of organic compounds:
- OLED screens: Organic molecules that emit light when electrified
- Battery electrolytes: Organic solvents enabling lithium-ion technology
- Touchscreen coatings: Fluorinated compounds repel fingerprints
6. Food Chemistry: The Science of Flavor and Nutrition
Why Food Tastes Like More
- Maillard Reaction: The beautiful browning of bread and steak (over 100 flavor compounds created)
- Capsaicin: Tricks your nerves into feeling the heat (Scoville scale measures this burn)
- Umami: Glutamate receptors explain why tomatoes and Parmesan taste savory
The Flavorists’ Secret Lab
Professional flavor chemists (like those at Givaudan or Firmenich) use organic chemistry to:
- Recreate natural flavors synthetically
- Develop new taste sensations
- Make foods healthier without sacrificing flavor
7. Environmental Challenges and Solutions
The Plastic Paradox
While plastics revolutionized modern life, their persistence creates problems. Organic chemists are developing:
- PLA plastics from cornstarch
- PHA bioplastics made by bacteria
- Chemical recycling methods to break plastics back into raw materials
Green Chemistry Principles
- Prevent waste rather than clean it up
- Design safer chemicals
- Use renewable feedstocks
- Increase energy efficiency
Companies like Patagonia and Interface use these principles to create sustainable products.
8. The Human Stories Behind the Science
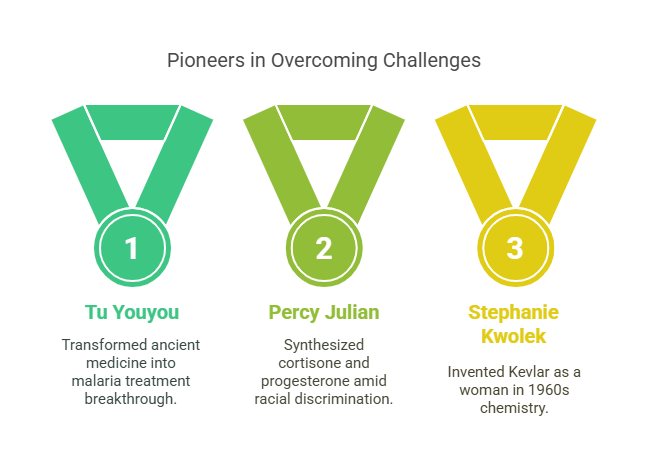
Overcoming Barriers
- Percy Julian: Synthesized cortisone and progesterone despite racial discrimination
- Tu Youyou: Turned ancient Chinese medicine into malaria treatment (2015 Nobel Prize)
- Stephanie Kwolek: Persisted as a woman in 1960s chemistry, invented Kevlar
The Role of Serendipity
Many discoveries came from accidents:
- Teflon: A failed refrigerant experiment
- Viagra: A heart medication with unexpected side effects
- Sweeteners: Saccharin discovered when a chemist forgot to wash his hands
Conclusion: Chemistry as a Human Adventure
Organic chemistry isn’t just about memorizing structures—it’s the ongoing story of human curiosity meeting carbon’s potential. From ancient alchemists to modern pharmaceutical researchers, we’ve been on a collective journey to understand and harness the power of molecular magic.
The next time you:
- Take medication
- Wear synthetic clothing
- Enjoy artificial flavors
- Recycle a plastic bottle
Remember—you’re experiencing the fruits of centuries of organic chemistry, a science that continues to shape our future in ways we’re only beginning to imagine.
FAQs about organic chemistry:
1. What’s the simplest organic compound?
Methane (CH₄) – just one carbon with four hydrogens. It’s natural gas, cow burps, and even Martian atmosphere!
2. Why does organic chemistry use so many hexagons?
Those are benzene rings – super-stable carbon arrangements found in everything from aspirin to gasoline. Their stability comes from “delocalized” electrons that act like a molecular security blanket.
3. How do organic chemists “see” molecules?
They use tools like:
- NMR (MRI for molecules)
- X-ray crystallography (molecular photography)
- Mass spectrometry (molecular fingerprinting)
4. What’s the most hated reaction by students?
Probably the Williamson ether synthesis – not because it’s hard, but because it’s often the first complex mechanism they learn!
5. Why do onions make you cry?
They release propanethial S-oxide, a volatile sulfur compound that reacts with eye moisture to form sulfuric acid (ouch!).
6. How did organic chemistry give us blue jeans?
Indigo dye (C₁₆H₁₀N₂O₂) was originally extracted from plants. Today, it’s synthesized in labs to color billions of jeans worldwide.
7. What’s the smelliest organic compound?
Ethyl mercaptan (C₂H₅SH) – added to natural gas so leaks smell like rotten eggs. Detectable at just 2 parts per billion!
8. Can you identify functional groups by taste?
Absolutely!
- -OH (alcohols): Burning (vodka)
- -COOH (acids): Sour (lemon juice)
- -CONH₂ (amides): Bitter (asparagus pee)
9. What organic compound do we make the most of?
Ethylene (C₂H₄) – over 200 million tons annually for plastics, antifreeze, and fruit ripening!
10. Why is organic chemistry called “orgo”?
Just student slang! Like “bio” for biology. (Bonus: Brits call it “org chem” – no cute nickname!)
11. How do glow sticks work?
Organic peroxides react with fluorescent dyes, releasing light without heat. Different dyes create different colors!
12. What’s the most expensive organic compound?
Pristane (C₁₉H₄₀), used in vaccines, costs ~$30,000 per gram – pricier than gold!
13. Why does bacon smell so good?
The Maillard reaction creates over 100 flavor compounds when amino acids and sugars react at high heat.
14. How did a chocolate bar help discover NSAIDs?
In 1948, a chemist licked his chocolate-covered fingers after handling ibuprofen precursors – noticing their bitter taste led to the discovery!
15. What’s the “bendy” molecule in flexible screens?
OLEDs (organic light-emitting diodes) use carbon-based polymers that emit light when electrified.
16. Why do mosquitoes bite some people more?
They’re attracted to specific skin volatiles like lactic acid and octanol – your personal chemistry cocktail!
17. What organic molecule makes champagne bubbly?
Carbonic acid (H₂CO₃) forms when CO₂ dissolves in wine, creating those festive fizz sensations.
18. How do fireflies glow?
Luciferin (C₁₁H₈N₂O₃S₂) reacts with oxygen in their tails, releasing cold light via chemiluminescence.
19. Why does cutting onions next to water help?
Water traps the volatile sulfoxides before they reach your eyes – kitchen hack meets organic chemistry!
20. What makes spicy food feel hot?
Capsaicin (C₁₈H₂₇NO₃) tricks your nerves into sensing heat where there’s none – a molecular prankster!
21. How did moldy bread lead to antibiotics?
Penicillin’s β-lactam ring (a strained 4-membered carbon-nitrogen structure) disrupts bacterial cell walls.
22. Why does ice float?
While not organic, it’s a great contrast: water’s hydrogen bonding creates open structures, unlike most organic liquids.
23. What’s the “molecular wheel” in nanotech?
Buckyballs (C₆₀) – soccer-ball-shaped carbon molecules used in drug delivery and solar cells.
24. Why does old paper smell good?
Lignin breakdown releases vanilla-scented vanillin and almond-like benzaldehyde – history’s perfume!
25. How is organic chemistry fighting climate change?
Through:
- Carbon capture (turning CO₂ into fuels)
- Biodegradable plastics (from corn or algae)
- Artificial photosynthesis (mimicking plants)
